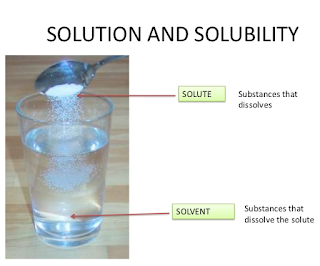
Solution
A solution is a homogeneous mixture of two or more
substances on molecular level. The constituent of the mixture present in a
smaller amount is called the Solute and the one present in a larger amount is
called the Solvent.
For example, when a smaller amount of sugar (solute) is mixed with
water (solvent), a homogeneous solution in water is obtained. In this solution,
sugar molecules are uniformly dispersed in molecules of water. Similarly, a
solution of salt (Na+ Cl–) in water consists of ions of salt (Na+, Cl–)
dispersed in water.
Types of Solutions (According to concentration or quantity of
solute)
33.
Saturated solution (High+ solute)
A stage of solution when the amount of solute is excess than concentrated solution the solution is called saturated solution.
A stage of solution when the amount of solute is excess than concentrated solution the solution is called saturated solution.







Comments
Post a Comment