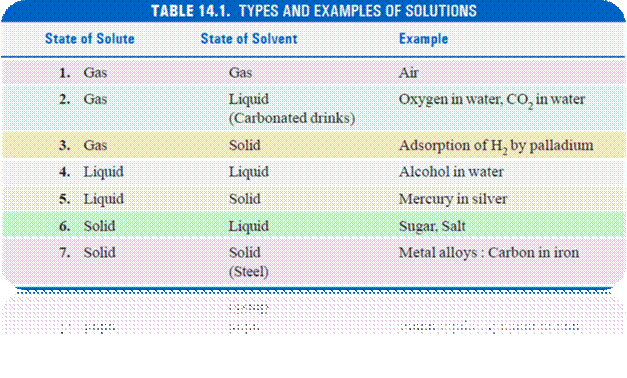
Concentration:> Mole fraction

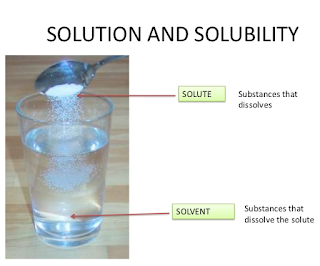
A simple solution is made of two substances: one is the solute and the other solvent. Mole fraction, X, of solute is defined as the ratio of the number of moles of solute and the total number of moles of solute and solvent. Thus, If n represents moles of solute and N number of moles of solvent, Notice that mole fraction of solvent would be, Mole fraction is unit less and